Research
Research Areas
Neuromorphic Semiconductor Bioelectronics
-
Bio-Inspired electronics
: Synaptic electronic (Neuromorphic electronics)
A human brain is composed of neuronal networks with ~1012 neurons connected by about 1 quadrillion (1015) synapses. Synapses conduct signals between neurons in an ever-changing manner. The effect of a signal transmitted synaptically from one neuron to another can vary enormously, depending on the recent history of activity at either or both sides of the synapse, and such variations can last from milliseconds to months. Activity-dependent changes in synaptic trans-mission arise from many mechanisms known collectively as synaptic plasticity.
The plasticity of the synapse is a key idea of human-brain memory formation and learning. Long-term changes in the transmission properties of synapses provide a physiological substrate for learning and memory, whereas short-term changes support a variety of computations.
We are interested in developing synaptic electronic devices based on organic semiconductor with low-power consumption, low cost, and high flexibility that mimics soft human organisms

1. Ion gel gated organic synaptic transistor with low energy consumption
In this study, we developed synaptic device based on organic semiconductor such as ion gel gated organic synaptic transistor (IGOST). At first, long-length one-dimensional polymer nanowire was formed in a desired position and direction by using an electrohydrodynamic nanowire printing system, and the resulting nanowire was used as a postsynaptic neuron, the artificial synapse device having a similar structure and function to the vital neuron was implemented.
Because one-dimensional nanowire-based artificial synapse devices have very small diameters of nanometers and structures similar to nerve fibers (very long lengths at the meter level), the implementation of highly integrated neuro- It is easy.
An ionic gel consisting of an ionic liquid and a polymer structure is used as a synapse connecting each neuron and an ionic molecule is used as a neurotransmitter to transmit a nerve signal on a principle similar to a biological synapse.
In addition, we report the low power dissipation similar to that of biological synapses through the formation of electrical double layer structures in ionic gels, but also the typical functions of synapses, such as excitatory post-synaptic current (EPSC), paired pulse facilitation spike-voltage dependent plasticity, spike-duration dependent plasticity, and spike-timing dependent plasticity (STDP).
It achieves a very low power consumption of 1.23 fJ per synaptic stimulus, which is one third of the power consumption of synaptic devices driven to date.


We also modulated synaptic property of IGOST by controlling the polymer characteristics. Semiconducting polymer channel of device is modulated its conductance by changing reduction/oxidation state according to ion doping. Applied gate voltage through the ion gel drives the ion into the polymer channel and device turns on. The ion intercalates into the polymer channel through the amorphous region made up with side chain and the ion was hindered its back-diffusion from the polymer by crystalline region. Therefore, we modulated the retention of ion doped state by adjust the polymer film fabrication conditions and controlling polymer side chain and backbone moieties. Enhanced crystallinity according to the annealing or self-assembly monolayer treatment enhanced the retention of doped state. Also, the adoption of specific moiety to the side chain such as glycol increase the capacity of ion capture. It improves the doping degree of channel. By controlling the synaptic property of device based on polymer science and engineering, we can apply the synaptic device into the memory device with proper operating conditions.

2. Organic Artificial Nerve System based on Synaptic Electronics
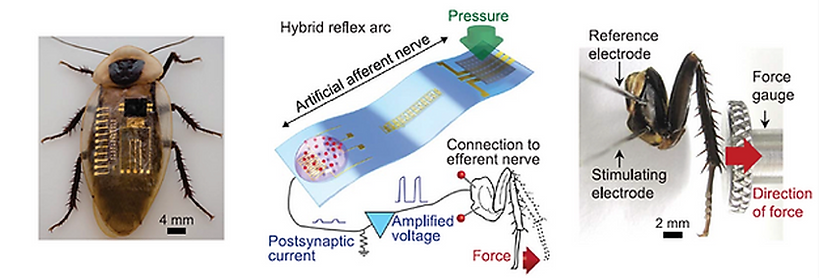
The somatosensory system's distributed network of receptors, neurons, and synapses efficiently process complex tactile information. We used flexible organic electronics to mimic the functions of a sensory nerve. Our artificial afferent nerve collects pressure information (1 to 80 kilopascals) from clusters of pressure sensors, converts the pressure information into action potentials (0 to 100 hertz) by using ring oscillators, and integrates the action potentials from multiple ring oscillators with synaptic transistor. Biomimetic hierarchical structures can detect the movement of an object, combine simultaneous pressure inputs, and distinguish braille characters. Furthermore, we connected our artificial afferent nerve to motor nerves to construct a hybrid bioelectronic reflex arc to actuate muscles. Our system has potential applications in neurorobotics and neuro-prosthetics.

We connected our artificial afferent nerve to the biological efferent nerves of a discoid cockroach (Blaberus discoidalis) to emulate a biological reflex arc. We used this hybrid system to demonstrate the flow of information from multiple pressure sensors through a neuromorphic circuit to deliver biomimetic postsynaptic oscillating signals into the biological efferent nerves in a detached cockroach leg, leading to the actuation of the tibial extensor muscle in the leg. The oscillating signals from our artificial afferent nerve elicit action potentials in nerves better than constant voltages. An increase in the amplitude and frequency of stimulation signals increases the number of activated muscle fibers and the forces generated by each muscle fiber, respectively. When we increased the intensity and duration of the stimulus application on the artificial afferent nerve, the maximum isometric contraction force of the tibial extensor muscle increased accordingly.
Furthermore, we report a stretchable neuromorphic implant based on a synaptic transistor that restores coordinated and smooth motions in the legs of mice with neurological motor disorders, enabling the animals to kick a ball, walk or run. This implantable stretchable neuromorphic efferent nerve (SNEN) can bypass a broken electrophysiological signal path and redirect electrophysiological signals to control body movement with soft neural interfaces and stretchable electronic systems in living organisms. An artificial synaptic transistor transmits the action potential (AP) from the gate electrodes into the excitatory post-synaptic current (EPSC) read by the drain electrode. Two synaptic transistors were individually connected to a flexor and an extensor to modulate leg motion. Also, a strain sensor based on carbon nanotube (CNT) detects the change in muscle length and enables a negative feedback loop in the implanted nerve system.

Inspired by the state-of-the-art understanding of bio-mimicking electronics, we are going to research neuromorphic bioelectronics that enables multimodal computing. The biomimetic hierarchical structures enable to collect the data from various sources such as sensors which detect the stimulations. Finally, our bio-inspired neuromorphic device must be used for biomedical engineering to ensure human health.
3. Organometal Halide Perovskite Artificial Synapses (Neuromorphic Electronics)
Herein, we fabricate and characterize an artificial synapse made from a bromine-containing OHP, CH3NH3PbBr3. This work represents the first attempt to apply OHP to an artificial synapse. The artificial synapse emulates important synaptic characteristics in a single electronic device.
Artificial synapses in a two-terminal structure of substrate/buffer-capped conducting polymer (BCCP) electrode/OHP/top electrode were fabricated to emulate important working principles of biological synapses. Metal-dot top electrodes emulate the presynaptic membrane at which presynaptic spikes are applied. Electrical pulses that are analogous to presynaptic spikes are applied to the top electrodes to induce ion migration in the OHP matrix to modulate the conductance of the thin film. Conductive paths form in the ion-rich OHP matrix to provide paths for ion migration and charge-carrier transportation; these emulate the synaptic cleft that allows transmission of neurotransmitters. The BCCP thin film and the conductive sublayer work together as a bottom electrode, which emulates the functions of the dendrites of a post-neuron to receive transient signals through the synaptic connection.

The conductance of OHP can be temporarily or persistently tuned by pulse-induced ion redistribution across the thin film, or ion injection into the BCCP layer to leave more vacancies. The BCCP layer could serve as a reservoir to trap mobile ions. These processes consecutively modulate the conductance of OHP thin film to realize multilevel state memory and thereby emulate the tunable synaptic response of natural systems
When a strong pulse or numerous pulses are applied, a fraction of ions can travel far enough to be trapped at the OHP/BCCP interface or even be injected into the BCCP and become trapped there. After the pulses, some ions drift back to their equilibrium positions, but some remain trapped at the interface and in the BCCP; consequently, some of the halide sites in the OHP are vacant to form conductive paths.
The increase in conductance can be maintained for a much longer time. Therefore, after the pulses, EPSC first decays due to drifting back of partial anions, but then the current level after this decay is maintained for a long time, due to the increased number of defect sites. This process emulates long-term potentiation in human memory. Due to the relatively low Ea of Br− (≈0.2 eV), it migrates easily under external pulses. Even though MA+ ions have much larger Ea (≈0.8 eV) than Br−, their possible migration cannot be fully excluded so they might also contribute to this mechanism.

The synaptic characteristics were realized by the consecutive modulation of electronic conductance resulting from the ionic migration mechanism in the organometal halide perovskite thin film. This is the first organic-inorganic hybrid perovskite artificial synapse. These properties present new resources for the development of neuromorphic electronics.
[References]
-
Advanced Materials, 32, 15, 1903558 (2020)
-
Science Advances, 2, e1501326 (2016)
-
Advanced Intelligent. System, 2, 2000012 (2020)
-
Science, 360, 998–1003 (2018)
-
Nature Biomedical Engineering, 6, 9 (2022)
-
Advanced Materials. 28, 5916-5922 (2016)


